Cleanroom classification & standards
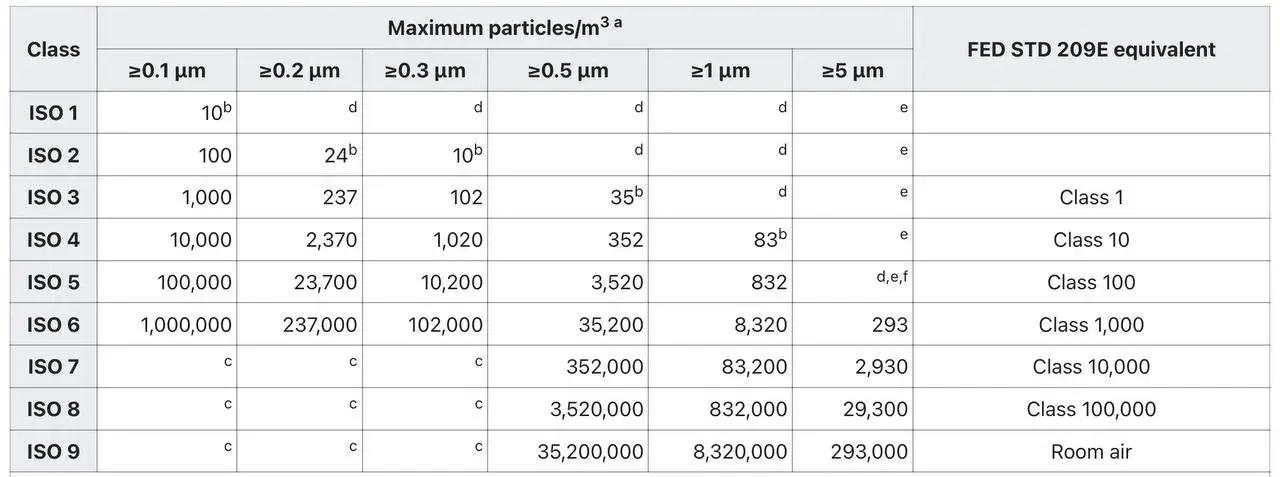
Cleanrooms are classified based on the level of cleanliness they achieve. The primary authority for cleanroom classification is the ISO 14644-1 standard, which defines various cleanroom classes. Let’s explore these classes:
1. ISO 1: The “cleanest” class, with the strictest particle limits.
2. ISO 2: Slightly less stringent than ISO 1.
3. ISO 3: Commonly used for critical manufacturing processes.
4. ISO 4: Suitable for semiconductor fabrication and pharmaceutical compounding.
5. ISO 5: Often referred to as a “Class 100” cleanroom, suitable for electronics assembly and sterile compounding.
6. ISO 6: Used in pharmaceutical production and biological research.
7. ISO 7: Provides a controlled environment for medical device manufacturing.
8. ISO 8: The least clean class, still significantly cleaner than a regular room.
Even the “dirtiest” class, ISO 9, maintains a higher level of cleanliness compared to a typical room. The equivalent Federal Standard 209E (FS 209E) classes are also commonly referenced1.
Cleanroom Standards
1. ISO 14644-1: This international standard specifies the classification of air cleanliness by particle concentration in cleanrooms and controlled environments. It covers both airborne particles and surface cleanliness.
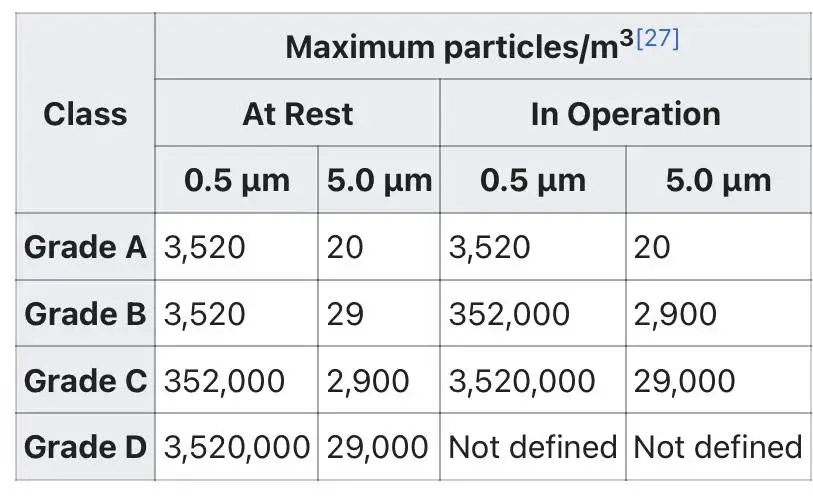
2. EU GMP (A-B-C-D): Applies specifically to pharmaceutical products and outlines cleanliness requirements for different stages of production.
3. USP (795, 797, and 800): Pertains to compounding pharmacies and ensures safe handling of medications.
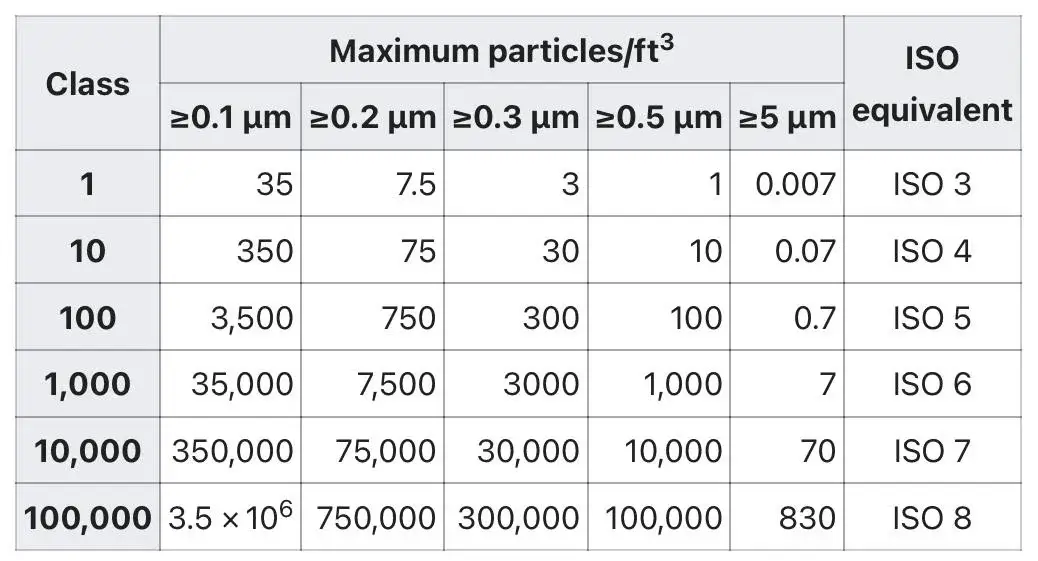
The Federal Standard 209E (FS 209E) used to be the standard in the US, but it was replaced by ISO 14644-1 in 1999. The old FS 209E classes correspond to ISO classes as follows:
• Class 10,000 (FS 209E) ≈ ISO 7
• Class 100,000 (FS 209E) ≈ ISO 8
Cleanroom Layout and Design
Achieving the desired cleanroom class involves careful planning. Consider the following factors:
• Square Footage: Allocate sufficient space for the clean zone and airlocks/gowning rooms.
• Airlocks: Limit the transition between classes to avoid skipping more than one class.
• Air Changes: Ensure adequate air changes per hour based on the process and room size.
Remember that cleanrooms must also adhere to industry-specific standards, such as EU GMP for pharmaceuticals and USP for compounding pharmacies.
If you’re designing a cleanroom, seek the expertise of a cleanroom specialist to create an optimal layout tailored to your specific needs
Cleanroom Training
Cleanroom Products
Cleanrooms projects we did
What is a Cleanroom?
Cleanroom classification & standards
Cleanroom validation & certification
Cleanroom air changes guidelines
Cleanroom FFU
How many FFU needed for a Cleanroom
Cleanroom PVC roll cut Flooring
Cleanroom Tbar Ceiling
Cleanroom airflow principle
Cleanroom ionization
Exdron Helmke Drum test kit
Exdron Body-Box Test